A clinical study is only as good as the data it collects. Validating your Electronic Case Report Forms (eCRFs)
ensures your study produces high quality data.
eCRF validation can be a major undertaking that requires experienced testers and takes weeks.
Even a small deviation can add weeks to your schedule for cleaning
up the data before statistical analysis can begin and actionable insights can be formed.
Our methodology converts your eCRF requirements into content for your study's validation package.
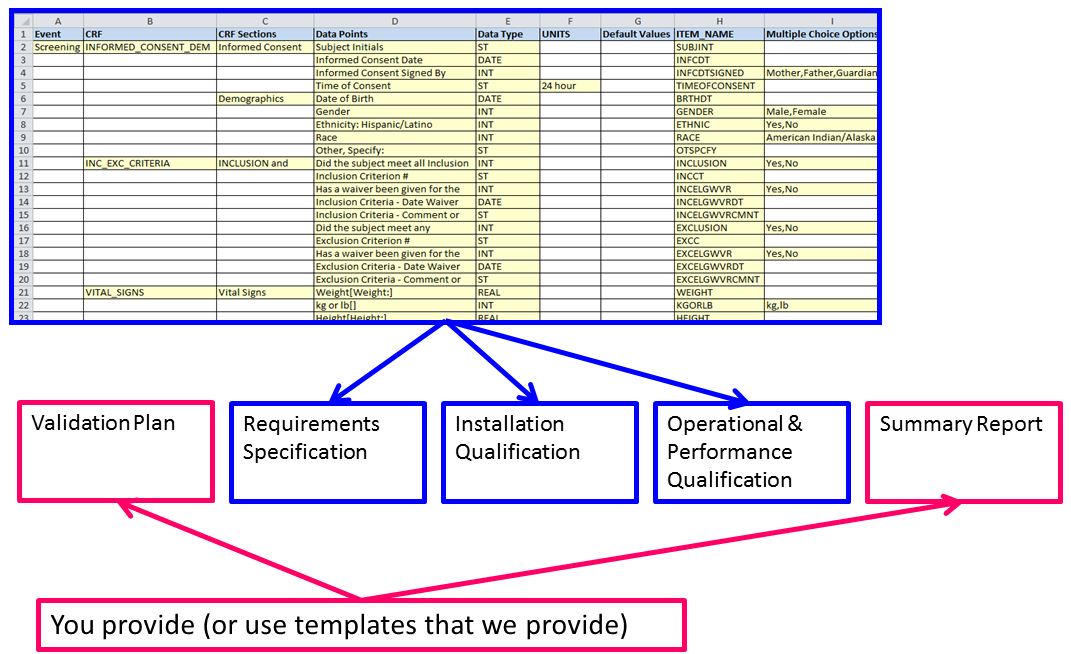
First, we teach you how to organize your data elements in an easy to use, easy to read manner. Our Data Collection Tool
makes sure we collect your Requirements Specification exactly as they were intended to be.
Second, we use advanced authoring software to create the content for your validation documents. Our software
uses your Requirements Specification (formatted in our template) to automate the creation of your Requirements Specification,
Installation Qualification, and Operational and Performance Qualification. By automating these 3 documents required by the
FDA, we reduce the amount of time it takes to begin testing and increase the quality because the test documents thoroughly
envelope your requirements.
Our clients used to plan for 4 to 5 weeks for drafting eCRF validation documents. Now, they plan 3 to 5 days. Contact us for references.
